Project 5: Systems and Circuits in MIA and Schizophrenia
Project Lead: Cam Carter, M.D.
Co-Investigators: Tyler Lesh, Ph.D.; Richard Maddock, Ph.D.; Martin Styner, Ph.D.; and Roza Vlasova, Ph.D.

Evidence from epidemiology implicates gestational maternal immune activation (MIA) in the pathophysiology of schizophrenia (SZ). These data, together with recent advances in our understanding of the role of immune molecules in normal brain development, have led to the overarching hypothesis of this Conte Center: that early activation of the maternal immune system alters brain development in offspring leading to structural and functional changes in connectivity that are associated with the emergence of psychopathology in adolescence and young adulthood. Our initial pilot studies of a unique nonhuman primate (NHP) MIA model found evidence of altered social, repetitive, and self-injurious behaviors that emerged during adolescence and were associated with increased striatal dopamine (DA). During the previous funding cycle, we found decreases in frontal cortical volumes and increases in extracellular free water (FW), a putative measure of neuroinflammation, measured using diffusion weighted MRI (DWI), in cingulate cortex gray matter (GM) in male MIA NHPs. These changes in the developing NHP brain were present as early as 6 months postnatally, prior to the emergence of any behavioral abnormalities, and highlight the importance of the early postnatal period for understanding the effects of MIA on brain development. In a parallel study in patients with SZ, we showed increased GM FW with maximal differences present in anterior cingulate, supporting the clinical relevance of the NHP MIA model. In the next round of study, we focus on the effects of MIA in the early postnatal period and on brain development in female NHPs, scanning both males and females at 6 and 18 months of age. To obtain an integrated cross-species understanding of the effects of MIA and SZ on the development of cortico-striatal functional circuitry, we will pursue the following three aims:
- Longitudinal imaging studies of sex effects on phenotypic heterogeneity in MIA NHPs,
- Computational model–based analysis of frontal-striatal circuitry associated with motivation and cognitive control in SZ, and
- Mechanisms underlying sex-related phenotypic heterogeneity in SZ.
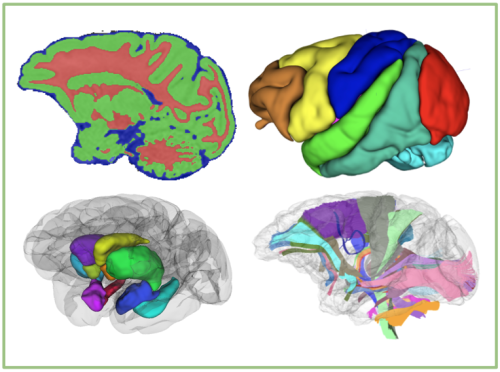
Interpretation of the results of these imaging studies will be enhanced by tissue analyses in the NHPs (Project 4) at age 18 months and measurement of the BIR and immunoreactivity of the mothers (Project 1) of the offspring we plan to image. Finally, in addition to the above study in MIA NHPs, and in line with our Center's expanded focus on MIA effects and of SZ on cortico-striatal circuitry, we will use a novel computational model–based fMRI analyses to dissect the neural circuitry underlying motivation and cognitive control deficits as well as their relationship to cortical FW and midbrain neuromelanin (NM), a novel proxy for hyperdopaminergic activity in SZ. This computational model will allow cross-species comparisons of the role for cortico-striatal circuitry in similar behavioral assays in the mouse MIA model. Finally, as in the NHP study, we will also examine the role of sex in the phenotypic heterogeneity in humans with SZ at the neural systems level and in relationship to symptoms and clinical outcomes.